
Fitbit gets FDA clearance for ECG app
The wearables maker received 510(k) clearance for an electrocardiogram app used to assess heart rhythm for atrial fibrillation.

The wearables maker received 510(k) clearance for an electrocardiogram app used to assess heart rhythm for atrial fibrillation.

Fitbit’s new, $330 smartwatch has a slew of new features, including a wrist-based temperature sensor and a sensor to monitor stress.

Break down the silos. Take control of your provider data.

Fitbit launched a large-scale study to validate the use of its technology to detect atrial fibrillation. The company plans to enroll hundreds of thousands of people into the virtual study.

A study launched by Johnson & Johnson is currently enrolling Medicare patients to see if an Apple Watch feature can reduce users’ stroke risk through the early detection of atrial fibrillation.

The algorithm is not designed to replace consumer-facing devices. But while most smart watches track electrical signals from the heart, Eko focuses on sound as a means to improve the work of front-line clinicians.

Dr. Joseph Wiesel claimed an Apple Watch feature for detecting atrial fibrillation violated his 2006 patent.

At a time when AI is reshaping pharma, Reverba Global CEO Cheryl Lubbert explained in an interview why empathy, context, and ethics still require a human touch.

The Canadian drugmaker was already considering putting itself up for sale after an FDA advisory committee voted against recommending approval for the drug, Brinavess, used for atrial fibrillation.

Software designed to detect atrial fibrillation - estimated to affect 8 million Americans this year - will be created for Fitbit and submitted to the FDA for approval.

Verily is being paid by iRhythm Technologies to develop a tool by improving existing hardware and software to effectively diagnose patients with asymptomatic atrial fibrillation.

According to findings presented at the American College of Cardiology's annual meeting, 0.5 percent of the more than 400,000 study participants received a notification about irregular pulse and 84 percent of participants who received irregular pulse notifications were found to be in atrial fibrillation at the time of the notification.

The San Francisco-based company plans to use the money primarily to fund its ongoing Phase II study of the drug, which it initiated in May.

Cardiac mapping has been an important tool to make ablations safe and effective, but it has required catheterization, unlike Medtronic's new product.
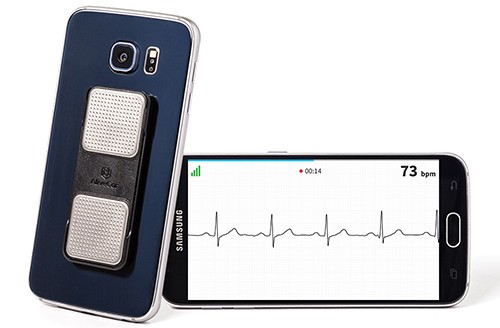
AliveCor Founder and CMO Dr. Dave Albert said that this opens the door to the NHS buying AliveCor devices for all 2 million atrial fibrillation patients in England "to save money and lives by preventing strokes."

Researchers from the Perelman School of Medicine at the University of Pennsylvania presented their study findings last week at the Heart Rhythm Society’s annual meeting in San Francisco.

Xerox‘s latest contribution to the world of healthcare could give doctors and patients an easier way to detect and monitor the most common type of arrhythmia using a basic webcam. The technology company built software algorithms that analyze the skin on the face for subtle color changes that could indicate irregular blood flow caused by […]