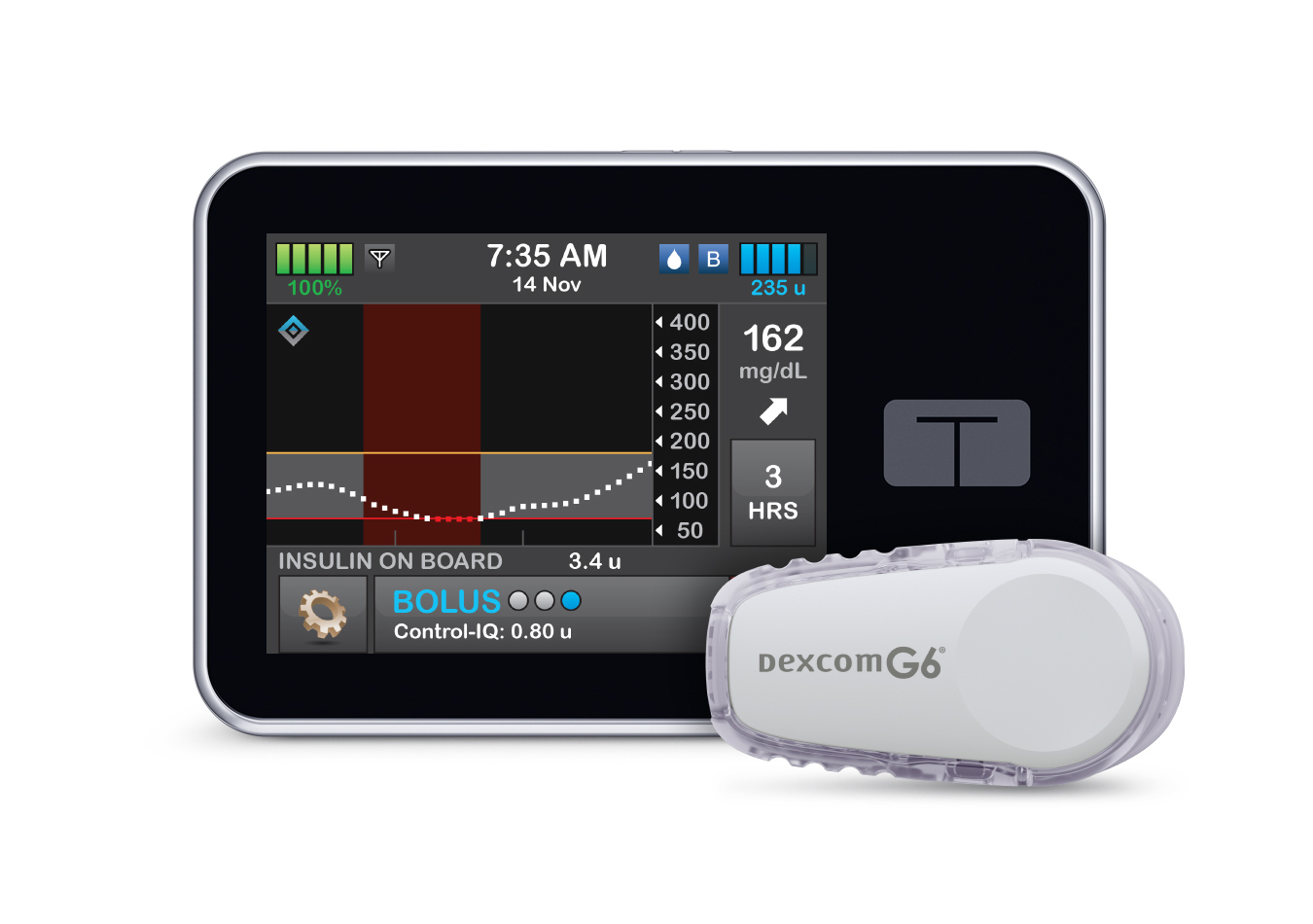
Tandem Diabetes snags FDA approval for interoperable insulin pump software
Tandem Diabetes’ technology will create a new approval pathway for predictive dosing software that can be used with other devices.

Tandem Diabetes’ technology will create a new approval pathway for predictive dosing software that can be used with other devices.

Scores of users took to Facebook to express their dismay, frustration and anger at Dexcom when the CGM device company failed to properly keep them updated after an IT outage prevented type 1 diabetes patients from sharing data.

Find Care, which was launched one year ago, was initially started to help patients find and identify location specific healthcare services through the Walgreens app and website.

On the day that an FDA official exhorts industry to develop interoperable diabetes devices, Medtronic announces that it is teaming up with a nonprofit group to do just that.

Fitabase CEO Aaron Coleman said he sees the integration being particularly useful to researchers looking at how behavioral recommendations in areas like physical activity and sleep can affect glucose levels to create more tailored care plans for diabetic patients.

San Diego-based Dexcom said at the annual J.P. Morgan Healthcare Conference in San Francisco that it's first-gen product co-developed by Alphabet's Verily may never get launched in the U.S.

Medtronic said the company will be rolled into its Diabetes Group to improve its diabetes management capabilities by adding Nutrino's comprehensive food database, food analysis system and nutrition-science expertise.

One episode shows how CMS evolved its thinking of how to cover continuous glucose monitoring when Medicare beneficiaries wanted to use it with their iPhones to share data with caregivers, family and physicians.

Months ahead of schedule, FDA clears the use of DexCom's latest continuous glucose monitoring system for patients two and up, freeing them from routine fingerstick calibration.
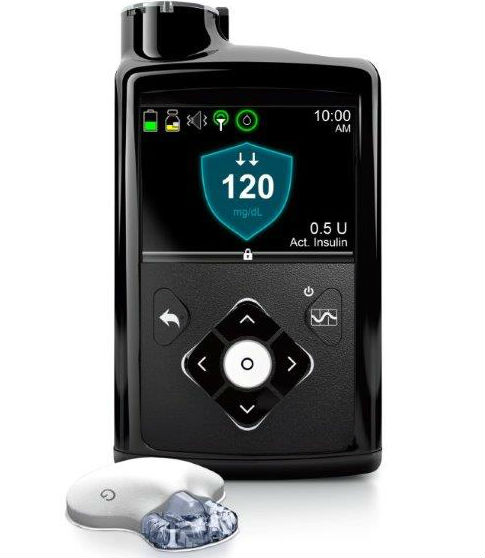
After a Type 1 patient penned a post on why she didn't think Medtronic's 670G hailed as the first artificial pancreas was all that, another patient who uses the device writes it's exactly that and then some.

As a type 1 diabetes patient, I have decided to stay on an open-source insulin delivery solution instead of switching to a hybrid closed-loop insulin delivery system from Medtronic whose FDA approval was widely hailed.
A sooner-than-expected availability of a rival's continuous glucose monitoring system to Medicare patients sends Dexcom's stocks lower, but an analyst believes this will be temporary.

Fitbit CEO and chairman James Park outlined the company's progress in a conference call late Wednesday to discuss the company's earnings in the third quarter.

Abbott struck a deal with Bigfoot Medical by which the latter will use Abbott's glucose sensing technology to develop an artificial pancreas and not that of DexCom, the CGM market leader.

CMS announced its decision to cover continuous glucose monitoring sytems for Medicare patients in January, a big win for Dexcom.