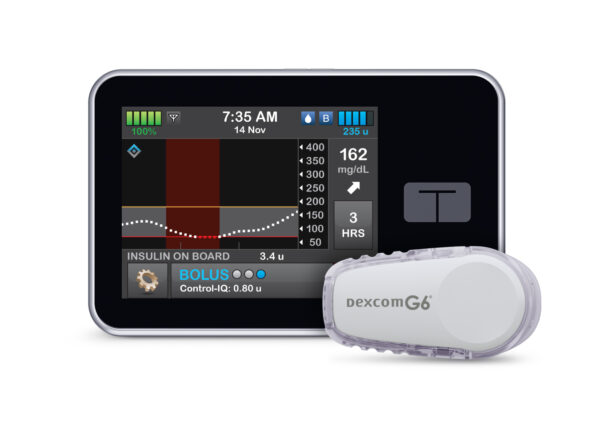
Tandem Diabetes Care received FDA approval for a new predictive dosing software that can work with Dexcom’s continuous glucose monitors.
Faced with growing competition in the market for diabetes devices, Tandem Diabetes Care’s stock shot up in February when the U.S. Food and Drug Administration classified its insulin pump as the first in a new device category for interoperable insulin pumps. The San Diego-based company got an early present in December when its newest technology, a predictive software designed to keep users within the optimal blood sugar range, received a de novo approval from the FDA.
The technology went through the FDA’s new interoperability pathway, which encourages device-makers to build products that can be used across systems or platforms. Both Tandem Diabetes’ software and its t:slim X2 insulin pump are designed to work with Dexcom’s continuous glucose monitors, with the ability to predict blood glucose levels 30 minutes in advance and increase or decrease insulin levels accordingly.
The Dec. 13 approval designated Tandem Diabetes’ software as the first device in a new category for interoperable automated glycemic controllers, paving the way for future approvals in the category.
In the future, other companies with equivalent technologies will be able to go through the FDA’s 510(k) review process, said Dr. Tim Stenzel, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health.
In a news release, Stenzel said the approval was a continuation of the FDA’s work “…to help ensure the safety and efficacy of innovative and customizable diabetes management systems that may help patients better tailor their treatments to their individual needs.”

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
The approvals this year marked good news for Tandem, after it recovered from a plummeting stock price two years ago as competitors took a larger slice of the market. In mid-2017, the company’s stock fell below $1 per share. Now, the company’ stock is valued at $60.53 per share.
“With this clearance, we will be launching the most advanced automated insulin dosing system commercially available in the world today,” Tandem Diabetes CEO John Sheridan said in a news release. “This is a testament to our commitment to improving the lives of people with diabetes by offering simple-to-use products that deliver superior performance.”
For the approval, Tandem Diabetes submitted the results of a randomized clinical trial that involved 168 participants with type 1 diabetes. Patients either used insulin pumps installed with Tandem’s new Control-IQ Technology controller, or they used a continuous glucose monitor and insulin pump without the controller.
According to the study, the controller was able to determine safe and effective insulin delivery with limited user intervention outside of mealtimes. The FDA also evaluated the controller’s ability to communicate with all parts of the system, as well as its reliability and cybersecurity measures.
“Not only do new closed-loop systems need to be effective at improving glycemic control, they must also be easy to understand and use so patients can experience the full benefits of the technology,” Boris Kovatchev, principal investigator for the trials and director of the University of Virginia’s Center for Diabetes Technology, said in a news release. “The t:slim X2 insulin pump with Control-IQ technology successfully achieved both objectives in the clinical studies.”
Tandem expects to make the new software available by the end of January.
Photo credit: Tandem Diabetes Care












